博雅辑因建立了国内首个GMP级别基因编辑临床应用中心
-

广州基地
- 已建成5200+平方米基因编辑细胞疗法中试基地
- 已具备大规模临床级别细胞治疗产品生产能力
-

完整、高效、稳定的生产能力和质量管理体系
- 放大、稳定的生产工艺,实现重要参数全流程保持稳定
- 具备疗效和安全性保障的基因编辑效率
-
国际认可
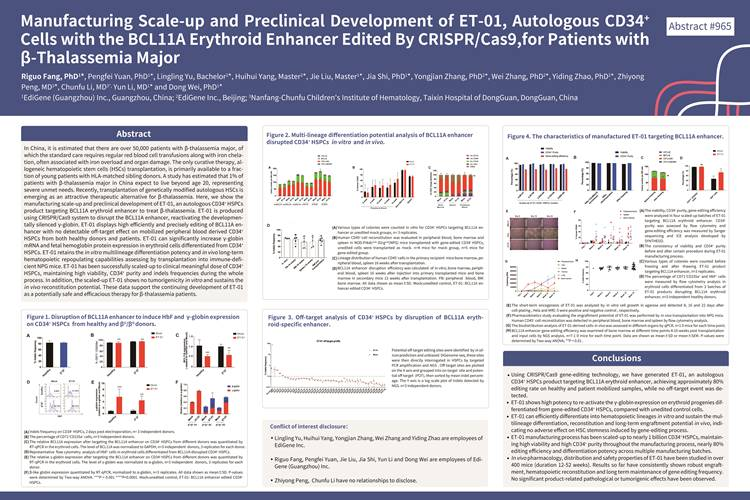
2019年12月第61届美国血液学年会(ASH)上发布β地中海贫血基因疗法项目(ET-01)的部分数据,重点介绍ET-01临床级规模化生产,以及支持其后临床试验的体外和体内的临床前研究的安全性和有效性数据。






