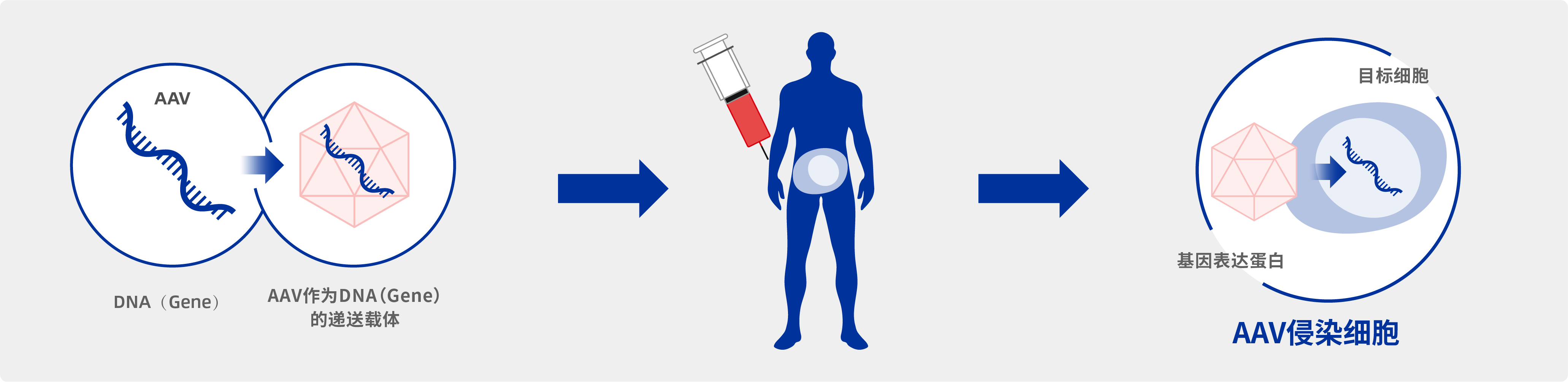
体内疗法:RNA碱基编辑平台
我们构建了将LEAPER™技术转化为体内编辑疗法的完整开发策略,包括疾病选择、目标突变选择、疾病模型的构建、arRNA设计与优化,配合相应的递送工具,以及相关的工具和技术。
领先项目I
Usher综合征(Usher syndrome)是一种罕见遗传性疾病,以先天性耳聋和进行性的视网膜色素变性为主要临床特征,全球每6,000人中就有1人患病。Usher综合征2型是最常见的亚型。USH2A基因突变是导致该亚型的主要原因。USH2A基因编码序列超过10KB,对于AAV或慢病毒递送系统来说太大;且该基因的突变范围很广。以至于目前既没有可行的治疗方案,也很难为该基因突变的患者开发基因替代疗法。
LEAPER™利用arRNA招募内源性ADAR蛋白实现对RNA的高效、精准编辑,递送也更加简单便捷。针对Usher综合征2型患者,LEAPER™能够在避免引入外源蛋白的情况下,在RNA水平上,对具有该疾病某些基因型的患者中,编辑和纠正突变蛋白成为正常USH2A蛋白,从而达到缓解症状,甚至治愈疾病的目标。
领先项目 II
黏多糖贮积症是由于溶酶体酶alpha-L-Iduronidase (IDUA)缺乏所导致的一种罕见的、可能致命的溶酶体储存缺陷。黏多糖贮积症I型(Hurler综合征)是黏多糖贮积症最严重的亚型。
目前,尚无针对该疾病的治愈方法。主要治疗手段包括改善生活质量的对症治疗、酶替代治疗和骨髓移植/造血干细胞移植,存在治疗手段及应用低效和困难等问题。
博雅辑因基于LEAPER™技术对IDUA信使RNA突变位点进行精准的、针对特定序列的RNA编辑,从而生成正常IDUA基因的信使RNA和蛋白质。数据显示,该技术在来自黏多糖贮积症I型患者的原代细胞中实现了显著、长效的IDUA酶活性。






